The differences between Fractionation, Hydrogenation and Esterification of oils and fats.
Fractionation, hydrogenation, and esterification are three key technologies for altering the physical and chemical properties of oils and fats to meet the diverse demands of the food industry. The fundamental difference among them lies in the distinct principles they employ to modify the properties of oils and fats. Below, we present a clear comparison of their differences through a table and detailed explanations.
Core Differences Summary
| Property | Fractionation | Hydrogenation | Esterification |
| Nature | Physical change | Chemical change | Chemical change |
| Principle | Separation based on the melting point differences of various triglycerides through cooling, crystallization, and filtration. | Adding hydrogen to the double bonds of unsaturated fatty acids under the action of a catalyst. | Rearranging the fatty acids on the glycerol backbone randomly or directionally under the action of a catalyst or enzyme. |
| Objective | Separating oils into high-melting-point (stearin) and low-melting-point (olein) fractions. | Increasing the melting point of oils to transform them from liquid to semi-solid or solid states; enhancing oxidative stability. | Altering the crystallization characteristics and plasticity of oils without changing the fatty acid composition. |
| Impact on Fatty Acids | No change in the chemical structure of fatty acids. | Change in the chemical structure of fatty acids: unsaturated fatty acids → saturated fatty acids; may generate trans fatty acids. | No change in the chemical structure of individual fatty acids, but a change in their distribution on the glycerol backbone. |
| Product Characteristics | Obtain two or more products with different physical properties (e.g., palm olein and palm stearin from palm oil). | Obtain hydrogenated oils with a harder texture and better stability. | Obtain oils with new melting curves and textures, such as trans-fat-free margarine and shortening. |
| Simple Analogy | Like leaving oil outside in winter, separating the liquid oil from the solidified part. | Like reinforcing unstable molecules to make them more "solid" and "stable". | Like shuffling a deck of cards (fatty acids) to get a new hand (new oil). |
Detailed Explanation
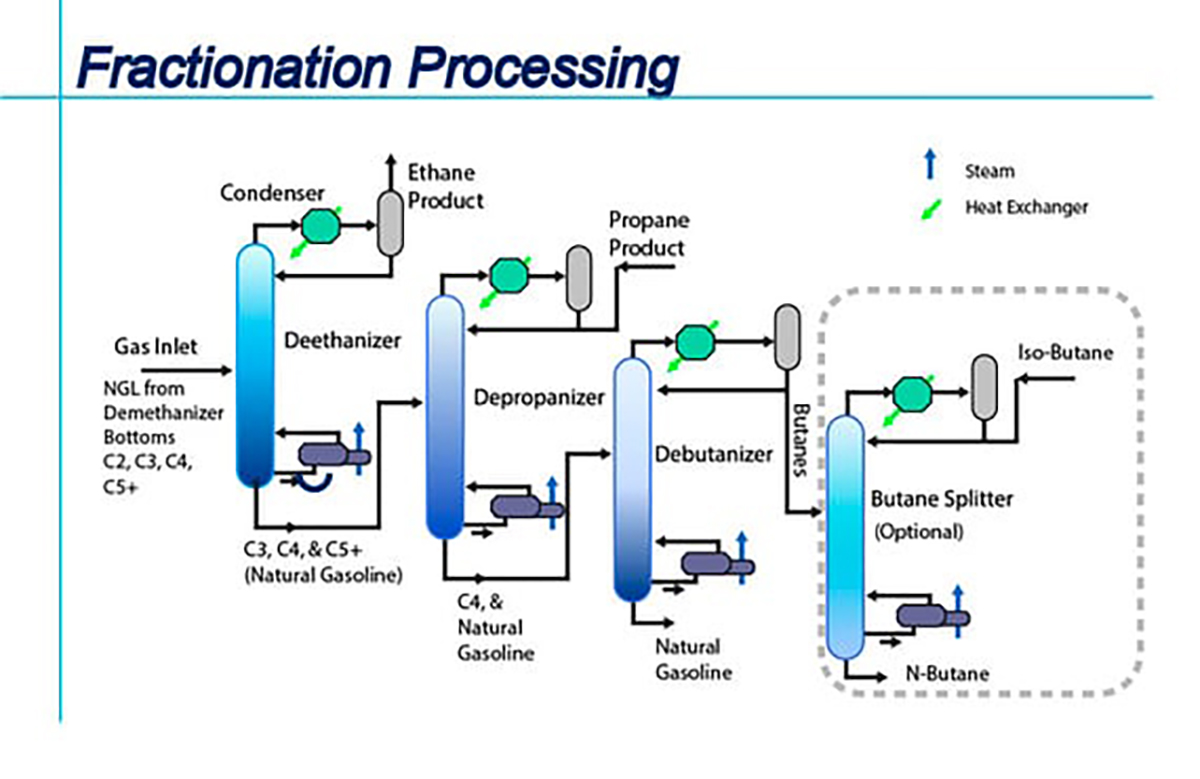
1. Fractionation
• Core idea: Separation, not alteration.
• Process: Slowly heat the oil to melt it, then cool it slowly at a specific temperature. The triglycerides with higher melting points will crystallize first, forming solid particles. These solid crystals (stearin) can then be separated from the still liquid oil (olein) through filtration or centrifugation.
• Application examples:
o Fractionation of palm oil: This is the most typical application of fractionation technology. Palm oil can be fractionated to obtain palm olein (used for cooking oil, frying oil) and palm stearin (used for margarine, shortening, and confectionery fats).
o Fractionation of butter: Produces purer butter fat, used for making high-quality pastries.
• Advantages: Pure physical process, no chemical changes introduced, no chemical reagents, and the product is natural.
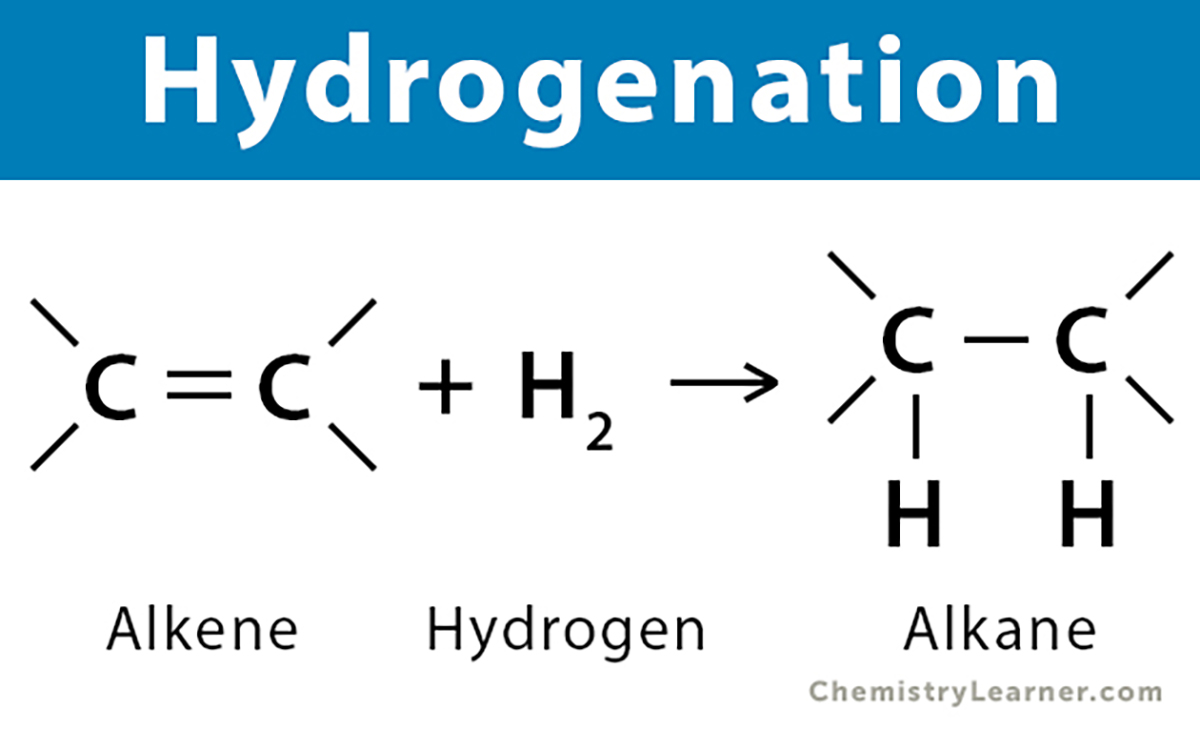
2. Hydrogenation
• Core idea: Add hydrogen to make the oil "harder" and "more stable".
• Process: Under high temperature, high pressure, and in the presence of a metal catalyst (usually nickel), hydrogen gas is passed into liquid oil. Hydrogen will add to the double bonds in the unsaturated fatty acid chains, reducing or eliminating the double bonds.
o Partial hydrogenation: Double bonds are not fully saturated, and a large amount of trans fatty acids are generated during this process. Due to the health hazards of trans fatty acids, it has been banned in many countries and regions.
o Complete hydrogenation: Double bonds are almost completely saturated, mainly generating saturated fatty acids (stearic acid), with almost no trans fatty acids. Completely hydrogenated oils are very hard and brittle, and usually need to be mixed with liquid oil or adjusted through ester exchange to modify their properties.
• Application examples:
o Manufacturing shortening and margarine: Transform liquid soybean oil, rapeseed oil, etc. into semi-solid form for baking and spreading.
o Improving oil stability: Extend the shelf life of frying oil and oil-containing foods.
• Disadvantages: Produces harmful trans fatty acids (partial hydrogenation) and loses essential fatty acids.
3. Ester Exchange
• Core idea: "Shuffling", changing the structure of triglycerides.
• Process: Under the action of a chemical catalyst (such as sodium methoxide) or lipase, the fatty acid glycerides in the oil molecules are "disassembled", and then the fatty acids are randomly or directionally recombined onto the glycerol backbone to form new triglyceride molecules.
o Random ester exchange: Fatty acids are randomly rearranged among all molecules.
o Directed ester exchange: Under specific conditions (such as controlled temperature), the rearrangement process is directed towards the desired direction.
• Application examples:
o Manufacturing trans-fat-free shortening and margarine: This is the most important modern application of ester exchange. By performing ester exchange between fully hydrogenated stearin (without trans acids) and liquid oil, a plastic fat with ideal texture and no trans fatty acids can be obtained.
o Improving the compatibility of cocoa butter substitutes.
o Altering the crystal structure of lard and butter to improve their performance in baking.
• Advantages: Can significantly change the physical properties of oils without generating trans fatty acids, making it a key alternative to partial hydrogenation technology. Summary
If you want to separate an oil into parts with different melting points, use fractionation. If you want to make a liquid oil harder and more stable, traditionally hydrogenation is used, but be aware of the issue of trans fatty acids. If you want to adjust the hardness, texture and plasticity of an oil without resorting to hydrogenation, which can produce trans fatty acids, then transesterification is the best choice. In modern oil industry, these three techniques are often combined to produce functional oil products that meet various specific needs.
Post time: Oct-14-2025